
Grade level: 8
Time required: 4 class periods
Cost: $25 for a water pump and misc hoses
MA frameworks: Physical science 4, 8.
This activity investigates the movement of conservative species such as dye or Chloride (Cl-) in moving water systems. Students set up a laboratory experiment and gather data. They then model the system using mass balance equations and compare the results in Excel. The data gathering segement uses LEGO Robolab Investigator. Overall use of this information allows them to discuss the movement of salt in a local waterbody. How long does it take to leave after being applied during a winter storm? How long would fish have to deal with it in their environment?
Link here for the Modeling Conservative Substances Worksheet.
Description of Euler's Method, the numerical method used to solve the salt mass balance equation (which is, in truth, a differential equation).
d[salt]/dt = -(Qout/V)*[salt], where Qout is the flow rate, V is the volume of the beaker (up to the top), and [salt] is the salt concentration. NOTE: seawater is approximately 15 g/L salt (15 ppt).


 Grade level: 7
Grade level: 7 Grade level: 8
Grade level: 8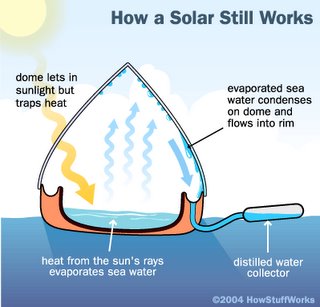 Grade level: 8
Grade level: 8 Scholars in Mr. Carpenito's science class can use these links to answer the questions on their Solar Desalination Challenge handout.
Scholars in Mr. Carpenito's science class can use these links to answer the questions on their Solar Desalination Challenge handout.